About Us
Dedicated to improving the lives of people impacted by diabetes

A century of promise. A future of hope.
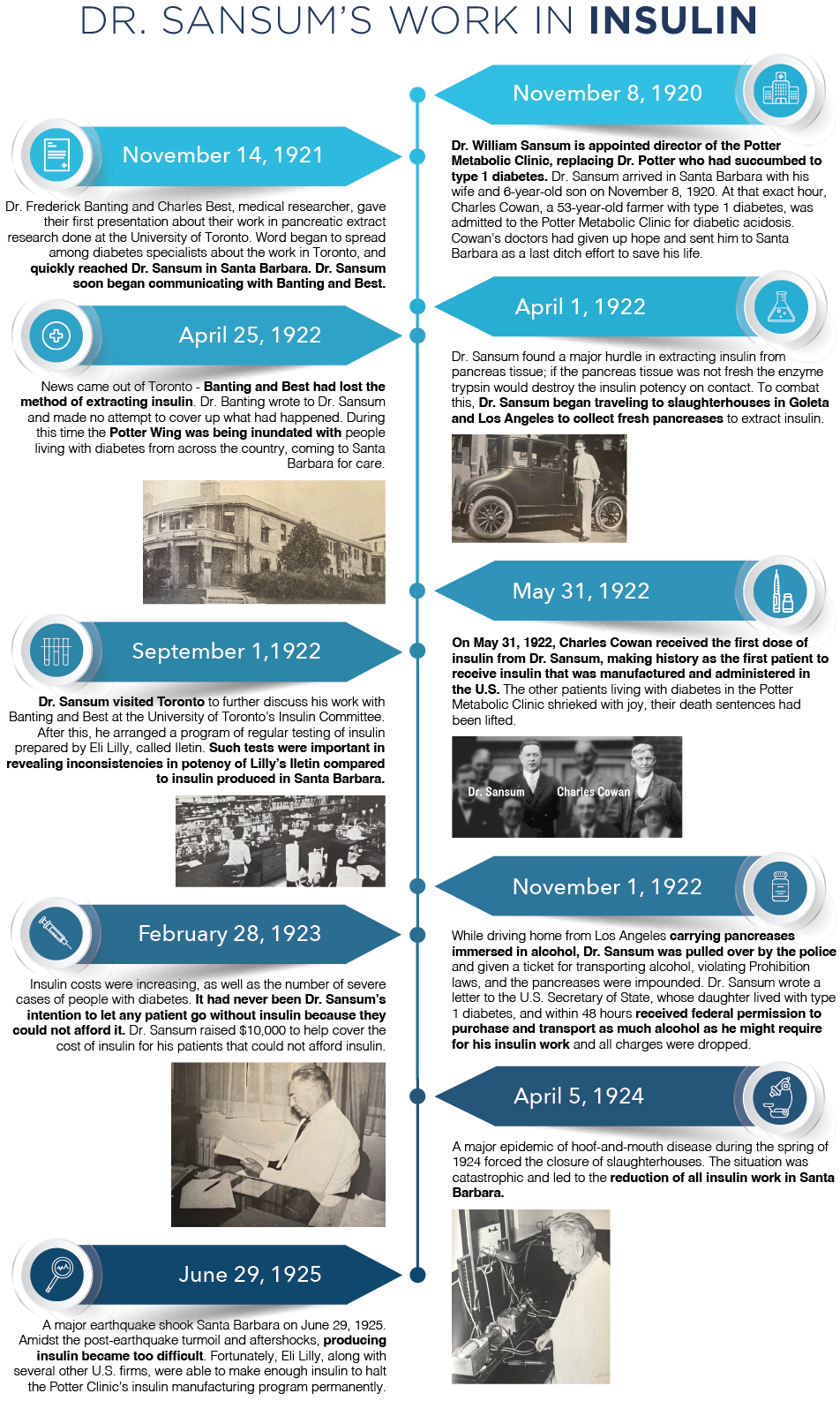
In 1922, our founder, renowned diabetes specialist Dr. William Sansum, profoundly changed the landscape for those living with diabetes by manufacturing and administering insulin for the first time in the United States.
Dr. Sansum’s crusade against diabetes led him to form SDRI in 1944 and established Santa Barbara as a center for advances in diabetes research. Today, SDRI continues to carry on Dr. Sansum’s legacy in diabetes glucose control and beyond, improving the health and lives of those impacted by diabetes.
SDRI’s legacy is also inextricably linked to Dr. Lois Jovanovic. Dr. Jovanovic served SDRI for 26 years, and was the former CEO and Chief Scientific Officer. Dr. Jovanovic was a visionary in modern-day diabetes care, and pioneered research and treatment guidelines that radically changed the global protocol for women with diabetes in pregnancy, resulting in uncomplicated pregnancies and healthy babies.
Dr. Jovanovic’s leadership and focus at SDRI led our investigators to enormous breakthroughs in the treatment of diabetes, including the development of the artificial pancreas technology. The artificial pancreas is a wearable device that measures blood sugar and automatically delivers the precise amount of insulin needed to maintain glucose control 24 hours a day. Today, SDRI continues research in artificial pancreas technology, participating in clinical trials. SDRI has conducted pivotal clinical research trials for all current FDA-approved systems.
As we look to the future of diabetes research, SDRI is proud to carry on Dr. Sansum’s and Dr. Jovanovic’s extraordinary legacies. We remember the words of our founder, “The outlook for medical research is brighter than at any time in history.” Dr. Sansum uttered that phrase over 100 years ago when he first arrived in Santa Barbara, but his words still ring true today.
We remain dedicated to improving the lives of the millions of people impacted by diabetes through research, education, and care.
A century of promise. A future of hope.
In 1922, our founder, renowned diabetes specialist Dr. William Sansum, profoundly changed the landscape for those living with diabetes by manufacturing and administering insulin for the first time in the United States.
Dr. Sansum’s crusade against diabetes led him to form SDRI in 1944 and established Santa Barbara as a center for advances in diabetes research. Today, SDRI continues to carry on Dr. Sansum’s legacy in diabetes glucose control and beyond, improving the health and lives of those impacted by diabetes.
SDRI’s legacy is also inextricably linked to Dr. Lois Jovanovic. Dr. Jovanovic served SDRI for 26 years, and was the former CEO and Chief Scientific Officer. Dr. Jovanovic was a visionary in modern-day diabetes care, and pioneered research and treatment guidelines that radically changed the global protocol for women with diabetes in pregnancy, resulting in uncomplicated pregnancies and healthy babies.
Dr. Jovanovic’s leadership and focus at SDRI led our investigators to enormous breakthroughs in the treatment of diabetes, including the development of the artificial pancreas technology. The artificial pancreas is a wearable device that measures blood sugar and automatically delivers the precise amount of insulin needed to maintain glucose control 24 hours a day. Today, SDRI continues research in artificial pancreas technology, participating in clinical trials. SDRI has conducted pivotal clinical research trials for all current FDA-approved systems.
As we look to the future of diabetes research, SDRI is proud to carry on Dr. Sansum’s and Dr. Jovanovic’s extraordinary legacies. We remember the words of our founder, “The outlook for medical research is brighter than at any time in history.” Dr. Sansum uttered that phrase over 100 years ago when he first arrived in Santa Barbara, but his words still ring true today.
We remain dedicated to improving the lives of the millions of people impacted by diabetes through research, education, and care.

Our collaborators









































Our collaborators










































Contact
2219 Bath Street
Santa Barbara, CA 93105
Phone: 805-682-7638
Fax: 805-682-3332
Patient Care: 805-682-4793
Tax ID # 95-1684086


